Therapy Approaches for STXBP1 Disorder
Introduction
STXBP1-related disorder is a rare neurodevelopmental condition. Patients who have been diagnosed with STXBP1-related disorder have a change in their STXBP1 gene. Research is being done on how to treat, and hopefully one day, cure STXBP1 disorder. This blog post will look at the therapeutic approaches being researched to treat this disorder.
STXBP1 disorder results when the STXBP1 gene is affected. This gene is very important as it serves quite a critical function in our brain. The gene produces the syntaxin-binding protein 1. This protein is key in neuron communication. Neurons communicate to others by releasing molecules called neurotransmitters through the gap between the neurons, known as the synapse. This is somewhat analogous to how humans communicate. Think of a person as a neuron. We usually talk to each other to communicate. The words that pass between us are somewhat similar to neurotransmitters. The distance between two people can be thought of as a synapse.
STXBP1 is one of several proteins in the SNARE complex. When one of these proteins does not function properly, it can cause problems. Individuals with a malfunctioning STXBP1 gene don’t produce enough syntaxin-binding protein 1. In turn, the release of neurotransmitters does not activate, creating issues with neuron function. STXBP1 disorders affect an estimated 1 in 30,000 people across the world.
Gene Therapies
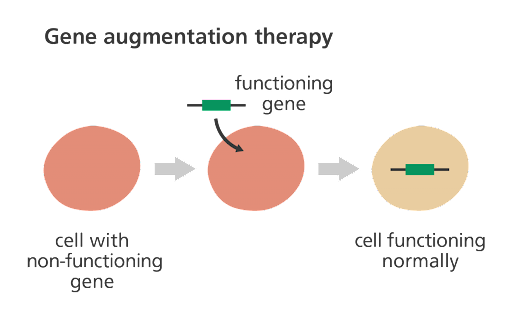
Researchers and scientists are trying to treat this disorder through precision therapies. One precision therapy approach is to target the genetic cause of the disorder: the STXBP1 gene. This can be done through gene therapies. Gene therapies have been gaining popularity within the past decades, as they have great potential. Gene therapy attempts to modify or manipulate a gene. In the case of STXBP1 disorders, individuals have a pathogenic mutation in one copy of the gene. As described above, this pathogenic mutation results in not enough syntaxin-binding protein 1 production, also called haploinsufficiency. Gene therapy could potentially fix this by replacing the gene with a normal version. There are other methods of gene therapy, however, due to the fact that STXBP1 is a haploinsufficiency disorder, gene replacement is currently considered the best approach.
One cutting-edge genetic therapy is CRISPR. CRISPR is something that has been around for a few years, but it is not fully understood yet. This technology can insert new sequences of DNA at certain spots, which could be applied to STXBP1. However, this technology is further down the line than current gene therapy technologies. Today, CRISPR is not perfect in targeting a specific gene, so there is concern it could make “off-target” changes with potentially severe consequences. Additionally, CRISPR may not be easily applied to neurological diseases. Delivery of CRISPR components to cells within the brain poses quite a challenge. Researchers are testing the viability of using CRISPR to treat neurological disorders by using the eye; the eye is directly connected to the brain, and could be a way to better access neurons.
Antisense Oligonucleotides
Another therapy that shows potential is antisense therapy. This therapy uses small molecules similar to DNA. These molecules are known as antisense oligonucleotides. These are small pieces of either DNA or RNA that can attach to molecules of RNA. This can block the RNA’s ability to make a protein work or function.
Image by Larissa Nitschke, Created with BioRender.
How does this apply to STXBP1? Well, antisense oligonucleotides(AOs) could be used to increase expression of the syntaxin protein 1, in order for messages to be sent across neurons as they normally would. This is where small molecules shine, as they aim to solve the precursor. In this case, they would increase expression of the protein rather than trying to lighten symptoms. A recent study has identified miR-218 and miR-424 as responsible for regulating STXBP1 expression, which is great! MicroRNA-218 and MicroRNA-424 are small pieces of RNA that can regulate gene expression, and are being researched in order to treat STXBP1.
Like gene therapies, ASOs are mainly being used for rare diseases; these genetic therapies tend to have high costs due to small patient numbers. Also, while ASOs have been successful in treating syndromes such as spinal muscular atrophy, they cannot always cross the BBB (blood-brain barrier), so intrathecal injection, injection into the spinal cord, may be used. Additionally, if ASOs target the wrong gene, there is a chance of side-effects. Finally, ASOs need to be administered multiple times in order to maintain therapeutic effects, which may make other forms of treatment more attractive. This aspect is different from gene therapies as gene therapies tend to be “one and done”.
Small Molecules
Another therapy approach that is being researched is small molecules. Small molecule therapy looks at processes occurring within the body. Researchers develop organic compounds that can either regulate or interfere with a malfunctioning process of the body. The drugs developed through this method are quite small in terms of molecular weight, allowing for more methods of delivery into the body.
Now how does this affect STXBP1 disorder and other rare diseases? Well, researchers look at the cause of the rare disease and try to find compounds that can correct a process that has gone awry. In STXBP1, therapy development has been focusing on seizures and neuron communication. Some advantages this therapeutic approach provides is it can be administered through multiple ways. Additionally, once a compound that can work has been found, it can be scaled pretty easily, which in turn makes it low in cost. One promising avenue in small molecule therapies is repurposed drugs, or drugs that have already been approved by the FDA or EMA for another condition. These would be faster to get to patients.
Chemical makeup of 4-phenylbutyrate
Today, a clinical trial is going on right now for 4-phenylbutyrate. 4-phenylbutyrate is a medication approved by the FDA used in removing ammonia from blood stream and excreting it. This medication is an example of drug repurposing.
Participants in this study are in the trial for 14 weeks. This study started in March of 2021. You can find more information about this trial here.
The STXBP1 Foundation has also funded a project to screen all FDA and EMA approved drugs to see if they might have an effect on STXBP1 disorder. Here is additional information on this topic.
Overall, therapy research is advancing for STXBP1 and other rare diseases. Treatments such as gene therapies, ASOs, and small molecules, are being heavily researched and more and more research is being done every passing year.
Sources/References
https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
https://www.jax.org/personalized-medicine/precision-medicine-and-you/what-is-crispr
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4251390/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7812771/
https://www.pharm-olam.com/blog/trends-in-small-molecule-rare-disease-research
https://www.brown.edu/news/2021-04-22/crispr-neuroscience
https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7355792/
https://www.frontiersin.org/articles/10.3389/fphar.2019.00305/full
https://clinicaltrials.gov/ct2/show/NCT04937062